Here are the rules for naming binary covalent compounds. A binary compound is one that consists of only two elements. The names are called systematic names. First, name the nonmetal furthest to the left and bottom of the periodic table by its element name. Second, name the other nonmetal by its element name, but shorten its name and add an -ide
Writing Names and Formulas for Compounds – ppt download
Science Chemistry Give the systematic name of each covalent compound. Spelling counts. P2S5: C3N4 Give the systematic name of each covalent compound. Spelling counts. P2S5: C3N4 BUY General Chemistry – Standalone book (MindTap Course List) 11th Edition ISBN: 9781305580343

Source Image: studylib.net
Download Image
The empirical formula has one Mg 2+ ion and one O 2− ion. Example 2.12.1 2.12. 1: Binary Ionic Compounds. Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. Ga 3+ and As 3−. Eu 3+ and O 2−. calcium and chlorine. Given: ions or elements.
Source Image: scientifictutor.org
Download Image
Covalent Compounds Contain 2 or more nonmetals. – ppt video online download Davidson Give the systematic name of each covalent compound. Spelling counts. SF,: Sulfur difluoride. Incorrect P,S3: Tetraphosphorus trisulfide CO: Carbon monoxide PN3: Triphosphorus pentanitride World of Chemistry, 3rd edition 3rd Edition ISBN: 9781133109655 Author: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste

Source Image: wikihow.life
Download Image
Give The Systematic Name Of Each Covalent Compound. Spelling Counts
Davidson Give the systematic name of each covalent compound. Spelling counts. SF,: Sulfur difluoride. Incorrect P,S3: Tetraphosphorus trisulfide CO: Carbon monoxide PN3: Triphosphorus pentanitride World of Chemistry, 3rd edition 3rd Edition ISBN: 9781133109655 Author: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste Aug 8, 2023The systematic names of the covalent compounds given are as follows: SF₆: This compound consists of one sulfur atom and six fluoride atoms. Its systematic name is Sulfur hexafluoride. P₄S₃: This compound consists of four phosphorus atoms and three sulfur atoms. Its systematic name is Tetraphosphorus trisulfide.
3 Ways to Name Covalent Compounds – wikiHow Life
CHEM 100: General Chemistry (O’Connor) 4: Covalent Bonding and Simple Molecular Compounds 4.2 Names and Formulas of Compounds – ppt download
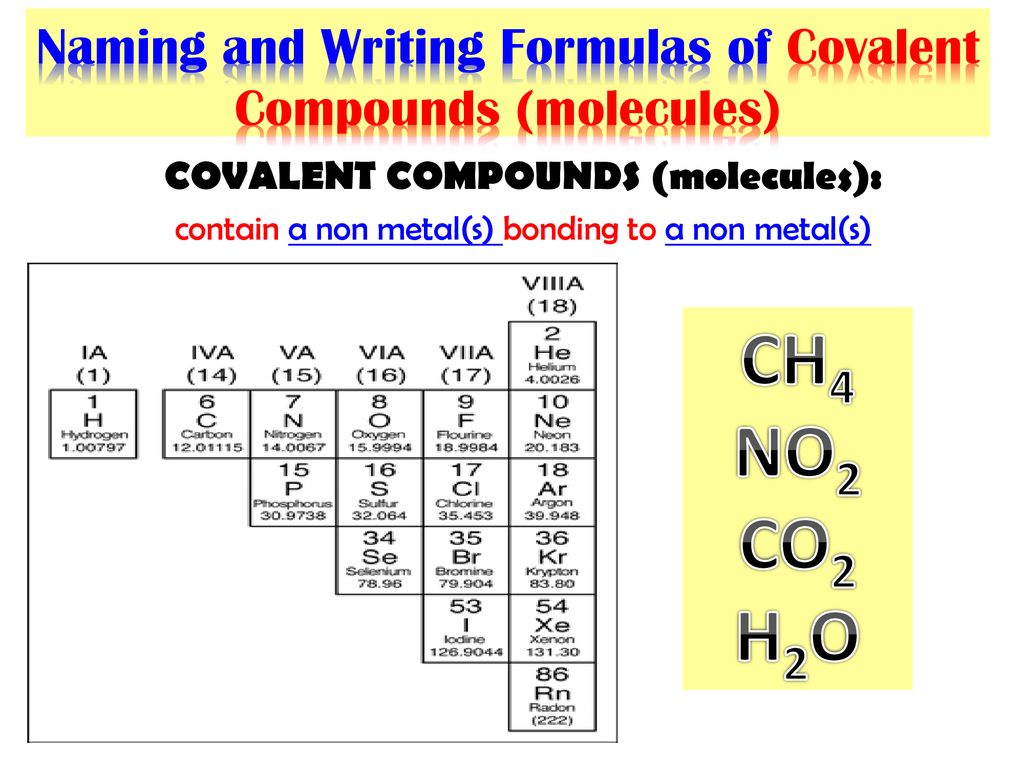
Source Image: slideplayer.com
Download Image
PPT – Naming Binary Covalent Compounds PowerPoint Presentation, free download – ID:5655505 CHEM 100: General Chemistry (O’Connor) 4: Covalent Bonding and Simple Molecular Compounds

Source Image: slideserve.com
Download Image
Writing Names and Formulas for Compounds – ppt download Here are the rules for naming binary covalent compounds. A binary compound is one that consists of only two elements. The names are called systematic names. First, name the nonmetal furthest to the left and bottom of the periodic table by its element name. Second, name the other nonmetal by its element name, but shorten its name and add an -ide

Source Image: slideplayer.com
Download Image
Covalent Compounds Contain 2 or more nonmetals. – ppt video online download The empirical formula has one Mg 2+ ion and one O 2− ion. Example 2.12.1 2.12. 1: Binary Ionic Compounds. Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair. Ga 3+ and As 3−. Eu 3+ and O 2−. calcium and chlorine. Given: ions or elements.

Source Image: slideplayer.com
Download Image
Chem – Naming Covalent Compounds Part 1 | Scientific Tutor Question Transcribed Image Text: Give the systematic name of each covalent compound. Spelling counts. SF: P,S,: SO: C;N4: Expert Solution Trending now This is a popular solution! Step by step Solved in 2 steps See solution Check out a sample Q&A here Knowledge Booster Learn more about Types of Bonds
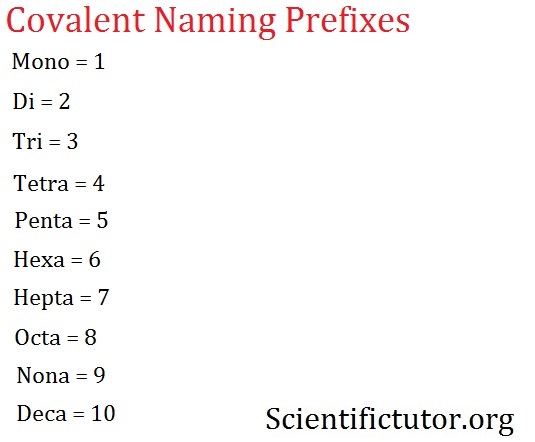
Source Image: scientifictutor.org
Download Image
Solved ment Score: 97.3% Resources [ Give Up? Feedback Resum | Chegg.com Davidson Give the systematic name of each covalent compound. Spelling counts. SF,: Sulfur difluoride. Incorrect P,S3: Tetraphosphorus trisulfide CO: Carbon monoxide PN3: Triphosphorus pentanitride World of Chemistry, 3rd edition 3rd Edition ISBN: 9781133109655 Author: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
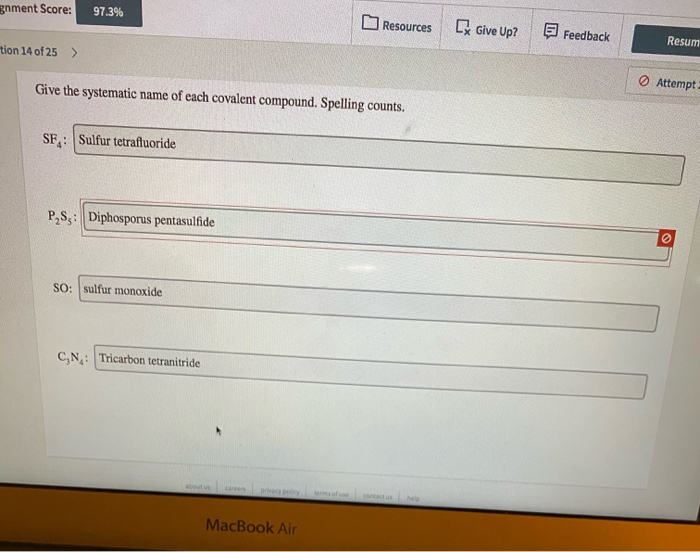
Source Image: chegg.com
Download Image
Nomenclature: Covalent Compounds | Texas Gateway Aug 8, 2023The systematic names of the covalent compounds given are as follows: SF₆: This compound consists of one sulfur atom and six fluoride atoms. Its systematic name is Sulfur hexafluoride. P₄S₃: This compound consists of four phosphorus atoms and three sulfur atoms. Its systematic name is Tetraphosphorus trisulfide.

Source Image: texasgateway.org
Download Image
PPT – Naming Binary Covalent Compounds PowerPoint Presentation, free download – ID:5655505
Nomenclature: Covalent Compounds | Texas Gateway Science Chemistry Give the systematic name of each covalent compound. Spelling counts. P2S5: C3N4 Give the systematic name of each covalent compound. Spelling counts. P2S5: C3N4 BUY General Chemistry – Standalone book (MindTap Course List) 11th Edition ISBN: 9781305580343
Covalent Compounds Contain 2 or more nonmetals. – ppt video online download Solved ment Score: 97.3% Resources [ Give Up? Feedback Resum | Chegg.com Question Transcribed Image Text: Give the systematic name of each covalent compound. Spelling counts. SF: P,S,: SO: C;N4: Expert Solution Trending now This is a popular solution! Step by step Solved in 2 steps See solution Check out a sample Q&A here Knowledge Booster Learn more about Types of Bonds